Answer:
4.7K
Step-by-step explanation:
Given parameters:
Initial volume = 75L
Initial pressure = 125psi
to atm gives 8.5atm
Initial temperature = 288K
New volume = 6.1L
New pressure = 25psi
to atm gives 1.7atm
Unknown:
New temperature = ?
Solution:
To solve this problem, we use the combined gas law which is given below:
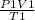 =
=
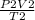
P, V and T are pressure, volume and temperature
1 and 2 are initial and new states
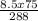 =
=
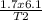
T2 = 4.7K