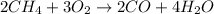Answer : The incomplete combustion equation of methane is, (B)
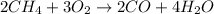
Explanation :
Combustion reaction : It is defined as the reaction in which a hydrocarbon react with oxygen to give carbon dioxide and water as a product.
As per question, the combustion of methane (a hydrocarbon) also gives carbon dioxide and water as a product.
The balanced combustion reaction of methane will be,
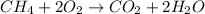
By the stoichiometry we can say that, 1 mole of methane react with 2 moles of oxygen to give 1 mole of carbon dioxide and 2 moles of water.
So, option (B) is an incomplete combustion reaction of methane and option (A) is the balanced combustion reaction of pentane.
Hence, the incomplete combustion equation of methane is, (B)