Step-by-step explanation:
A molecule with a triple bond will have a linear geometry as the angles between the bonds will be 180 degrees. Hence, it will be a linear geometry.
For example, a molecule of dinitrogen (
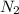 ) has nitrogen atoms bonded through triple bond as follows.
) has nitrogen atoms bonded through triple bond as follows.
:N ≡ N:
Since valency of nitrogen is 5 therefore, each nitrogen will have one lone pair of electrons.