Answer:

Step-by-step explanation:
The original equation is:
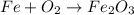
We notice that:
- we have 1 atom of Fe on the left, and 2 atoms of Fe on the right
- we have 2 atoms of O on the left, and 3 atoms of O on the right
Therefore, the equation is not balanced.
In order to balance it, we can add:
- a coefficient 3 in front of

- a coefficient 2 in front of
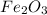
So we have:
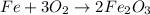
Now the oxygen is balanced, but the iron it not balanced yet, since we have 1 Fe on the left and 4 on the right. Therefore, we should add a coefficient 4 on the Fe on the left:
