Step-by-step explanation:
Formula mass of a compound is calculated by taking the sum of atomic masses of all the elements present in the compound.
Since, atomic mass of Na = 23 g/mol
Atomic mass of S = 32 g/mol
Atomic mass of O = 16 g/mol
Hence, formula mass of
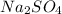 will be as follows.
will be as follows.
Formula mass =
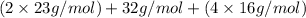
= 142 g/mol
Thus, we can conclude that formula mass of
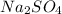 is 142 g/mol.
is 142 g/mol.