Answer:- 537 kJ of heat is released.
Solution:- For the given equation,
 is -657 kJ and the coefficient of
is -657 kJ and the coefficient of
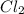 in the balanced equation is 2. It means 657 kJ of heat is released when 2 moles of chlorine are used. We need to calculate the heat released when 116 g of
in the balanced equation is 2. It means 657 kJ of heat is released when 2 moles of chlorine are used. We need to calculate the heat released when 116 g of
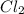 are used.
are used.
Grams of chlorine are converted to moles and then multiplied by the
 value and divided by the coefficient of chlorine and the set could be shown using dimensional analysis as:
value and divided by the coefficient of chlorine and the set could be shown using dimensional analysis as:

= 537.46 kJ
If we use the correct sig figs then it needs to be round off to three sig figs as the given grams of chlorine has only three sig figs. So, 537 kJ of heat is released.