Answer : The electronic configuration of chlorine (Cl) in noble gas notation will be:
![[Ne]3s^23p^5](https://img.qammunity.org/2019/formulas/chemistry/high-school/4hwjofwetiiiju8hlj98hs8an4c3qraui4.png)
Explanation :
Electronic configuration : It is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom are determined by the electronic configuration.
Noble-Gas notation : It is defined as the representation of electron configuration of an element by using the noble gas directly before the element on the periodic table.
Chlorine is the non-metal that belongs to group 17 and has 17 electrons in their shell.
The electronic configuration of chlorine (Cl) will be:
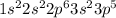
The electronic configuration of neon (Ne) will be:
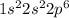
So, the electronic configuration of chlorine (Cl) in noble gas notation will be:
![[Ne]3s^23p^5](https://img.qammunity.org/2019/formulas/chemistry/high-school/4hwjofwetiiiju8hlj98hs8an4c3qraui4.png)