Answer:
The unknown substance is made up titanium.
Step-by-step explanation:
Density is defined as mass present in the unit volume of the substance.It is an intensive property.
Density of Aluminium =
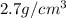
Density of copper =
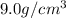
Density of iron =
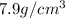
Density of titanium =
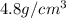
Density of the unknown substance:
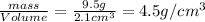
The density of the unknown substance is close to the density of the titanium. This means that unknown substance is made of titanium.