Answer:
1) The amount is less than one mole, 2) The jar is not completely filled, 3) We have 0.166 moles of sulfur.
Step-by-step explanation:
We proceed to respond each question below:
1) Is this amount more or less than one mole?
According to the Avogadro's Number, one mole of an element contains about
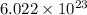 atoms. Given that we have
atoms. Given that we have
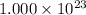 atoms, then we conclude that this amount is less than one mole.
atoms, then we conclude that this amount is less than one mole.
2) Place the jar underneath the counter. Was the jar completely filled?
Let suppose that the jar can contain up to a mole of sulfur. Then, we conclude that jar is not completely filled.
3) How many moles do you have?
The amount of moles is determined by dividing the current amount of atoms by Avogadro's Number:
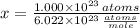
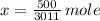
We have 0.166 moles of sulfur.