Answer:
C)0.19 mol/L .
Step-by-step explanation:
Hello!
In this case, since the chemical reaction between sodium hydroxide and sulfuric acid is:
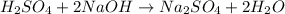
Whereas a 1:2 mole ratio is seen between the acid and the base, we can write:
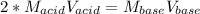
In such a way, we solve for the concentration of base and plug in to obtain:
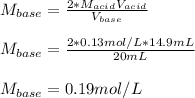
Thus, the answer is C)0.19 mol/L
Best regards!