Answer :
Single-displacement reaction : It is a type of reaction in which a more reactive metal displace the less reactive metal from the solution.
General equation for single-displacement reaction is,
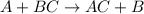
In this reaction, A displaces B because A is more reactive than B.
These reactions answered on the basis of reactivity series. The reactivity series image is shown below.
- Zinc react with copper sulfate
The balanced reaction is,
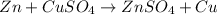
When zinc react with copper sulfate then zinc displaces the copper from copper sulfate because zinc is more reactive metal than the copper.
- Aluminium react with copper sulfate
The balanced reaction is,
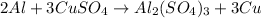
When aluminium react with copper sulfate then aluminium displaces the copper from copper sulfate because aluminium is more reactive metal than the copper.
- Zinc react with silver nitrate
The balanced reaction is,
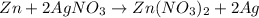
When zinc react with silver nitrate then zinc displaces the silver from silver nitrate because zinc is more reactive metal than the silver.
- Copper react with silver nitrate
The balanced reaction is,
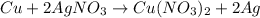
When copper react with silver nitrate then copper displaces the silver from silver nitrate because copper is more reactive metal than the silver.