Question 1
We start with the half reaction for silver (Ag) is
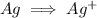
There is an imbalance of charge with left hand side having a charge of 0 and right hand side having a charge of +1. So we add an electron on the right hand side to balance the charges.
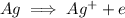
This would be the oxidation half reaction since
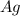 is being oxidized. Oxidation is loss of electrons.
is being oxidized. Oxidation is loss of electrons.
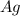 loses electrons to form the
loses electrons to form the
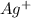
The next half reaction is
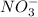

There are 3 oxygen atoms on left hand side and 1 on right hand side. So we add 2 water molecules on right hand side to balance oxygen.
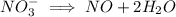
Then we add
 ions on left hand side to balance hyrdogen.
ions on left hand side to balance hyrdogen.

We have imbalance of charge with left hand side having an overall +3 (-1 + 4) while right hand side has 0. So we add 3 electrons on left hand side to get the charge to equal on each side.
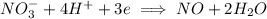
The
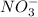 ion is getting reduced because it is gaining electrons. Reduction is gain of electrons.
ion is getting reduced because it is gaining electrons. Reduction is gain of electrons.
Question 2
We start with the half reaction of
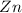
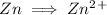
There is an imbalance of charge so we add 2 electrons on right hand side to balance the charge.
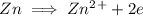
Zn is being oxidized in the reaction since it is losing electrons to obtain a higher oxidation state.
The half reaction for
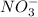 is similar as the one obtained above
is similar as the one obtained above
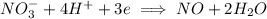
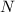 is being reduced since it is moving from a +5 oxidation state to a +2 oxidation state by gaining electrons.
is being reduced since it is moving from a +5 oxidation state to a +2 oxidation state by gaining electrons.
Question 3
We start with the dichromate ion
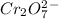
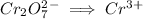
First we balance the chromium ions by adding a coefficient of 2 on right hand side.

Then we add 7 water molecules on right hand side to balance the oxygen
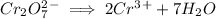
Now we add 14H^+ ions on left hand side to balance hydrogen

The we add 6 electrons on left hand side to give a +6 charge on each side
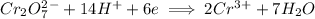
The chromium ion is being reduced since it moves from oxidation state of +6 to a +3 oxidation state.
The second half reaction:
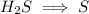
We balance the hydrogen by adding H^+ ions on right hand side
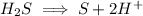
Now we balance the charge by adding 2 electrons on right hand side
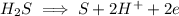
Hydrogen is being oxidized since it moves from oxidation state of 0 to an oxidation state of +2