Answer:- 0.143 M
Solution:- HCl and NaOH reacts in 1:1 mol ratio as shown in the below reaction:
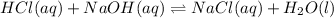
Let's calculate the initial moles of HCl and the moles of NaOH added to it:
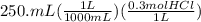
= 0.075 mol HCl
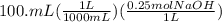
= 0.025 mol NaOH
Since they react in 1:1 mol ratio, 0.025 mol of NaOH will react with 0.025 moles of HCl.
Remaining moles of HCl = 0.075 - 0.025 = 0.050
Total volume of the resulting solution = 0.250 L + 0.100 L = 0.350 L
So, the concentration of HCl in the resulting solution =
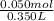
= 0.143 M
Hence, the concentration of HCl acid in the resulting solution is 0.143 M.