Here we have to get the the
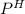 of the solution containing HOBr and KOBr.
of the solution containing HOBr and KOBr.
The
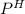 of the solution is 8.47
of the solution is 8.47
The mixture of HOBr and KOBr is a buffer solution. The
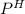 of the buffer solution is determined by the Henderson equation which is:
of the buffer solution is determined by the Henderson equation which is:
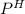 =
=
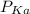 + log
+ log
![([salt])/([acid])](https://img.qammunity.org/2019/formulas/chemistry/high-school/bjo57cy92jky5jy3yg5ds7pysuyorp8hkz.png)
Here [salt] i.e. KOBr is 0.30 mol/L and [Acid] is 0.50 mol/L.
The
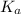 value of HOBr is 2×10⁻⁹.
value of HOBr is 2×10⁻⁹.
We know,
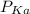 = -log
= -log
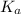
Or,
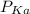 = -log (2×10⁻⁹)
= -log (2×10⁻⁹)
Or,
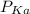 = 8.698
= 8.698
On plugging the values:
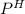 = 8.698 + log
= 8.698 + log
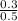
Or,
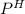 = 8.698 - 0.221
= 8.698 - 0.221
Or,
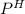 = 8.47
= 8.47
Thus the
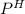 of the solution is 8.47.
of the solution is 8.47.