Answer: Option (d) is the correct answer.
Step-by-step explanation:
An ion is a specie that contains either a positive or a negative charge.
For example,
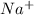 ,
,
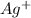 ,
,
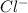 ,
,
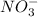 , etc are all ions.
, etc are all ions.
Therefore, an ionic equation is defined as an equation that contains participating dissolved species in the form of ions.
For example,
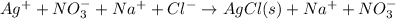
Here, only AgCl is the precipitate as it is insoluble but rest of the species are ions as they have dissolved.
Thus, we can conclude that a complete ionic equation an equation showing all dissolved compounds as ions.