Answer: The correct answer is Option A.
Step-by-step explanation:
We are given:
Density of a gas = 1.25 g/L
To convert this quantity into ounce/mililiter, we use the following conversion factors:
1 gram = 0.04 ounce
1 Liter = 1000 mili Liter
Converting the given quantity of gram/Liter into ounce/mililiter, we get:
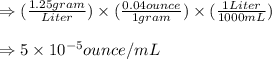
For the conversion, we multiply 1.25 by 0.04 and then divide the result obtained by 1000.
Hence, the correct answer is Option A.