Answer: Option (C) is the correct answer.
Step-by-step explanation:
When an electron is transferred from one atom to another then it results in the formation of ionic bond. Whereas when there is sharing of electron(s) then it results in the formation of covalent bond.
Chlorine being a non-metal readily accepts electron from a metal. For example, NaCl is an ionic compound.
Also, chlorine is able to combine with another chlorine atom by sharing of an electron. For example,
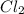 is a covalent compound.
is a covalent compound.
Thus, we can conclude that a chlorine atom can form ionic bonds by accepting an electron and covalent bonds by sharing electrons.