Answer:

Step-by-step explanation:
To convert from grams to moles, we must use the molar mass.
1. Molar Mass
Use the Periodic Table to find the masses of the individual elements (silicon and oxygen) in sand.
- Silicon (Si): 28.085 g/mol
- Oxygen (O): 15.999 g/mol
Examine the formula for sand: SiO₂. There is a subscript of 2 after oxygen, so there must be 2 oxygen atoms. Multiply oxygen's mass by 2 and add silicon's mass to find the molar mass of sand.
- SiO₂: 2(15.999 g/mol) + 28.085 g/mol= g/mol
2. Calculate Moles
Use the molar mass as ratio.
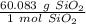
Multiply by the given number of grams (30)
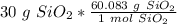
Flip the fraction so the grams of sand will cancel.
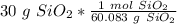
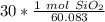
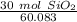
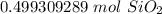
3. Round
The original measurement of grams has 1 signfiicant figure. We must round our answer to 1 sig fig.
For the answer we found, that is the tenth place. The 9 in the hundredth tells us to round the 4 to a 5.
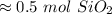
There are about 0.5 moles of SiO₂ in 30 grams.