Answer:
B. Measure the mass of the gases in each container.
Step-by-step explanation:
Hello,
In this case, we take advantage of the ideal gas equation in terms of mass and atomic mass as shown below:
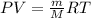
Since the scientist does not know which gas is at each container, one strategy to find it out is by measuring the mass of the gases in each container and as the pressure, volume and temperature are known, one computes the molar mass to subsequently identify each gas based on its atomic mass, based on:
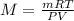
Thus, once one computes the molar mass of each container, one identifies where is each gas, therefore, the answer is B. measure the mass of the gases in each container.
Best regards.