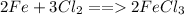The correct answer is D. Using the law of conservation of mass the number of atoms on each side of the equation should be equal. Through introspection, we find that there are 2
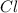 atoms on reactant side as opposed 3Cl atoms on product side. If we add a coefficient of 3 on
atoms on reactant side as opposed 3Cl atoms on product side. If we add a coefficient of 3 on
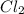 we get
we get
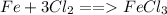 .
.
Now there are 6Cl atoms on reactant side and 2 on product side, hence we add a coefficient of 2 on both
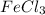 and
and
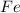 . The balanced chemical equation is,
. The balanced chemical equation is,