Answer:
a) Moles of ibuprofen in a bottle of ibuprofen = 0.47 mol
b) No. of molecules in a bottle of ibuprofen is

Step-by-step explanation:
a)
Given:
Molar mass of ibuprofen = 206.3 g/mol
Amount of ibuprofen in one tablet = 200 mg
No. of tablet in a bottle = 485
Total amount of ibuprofen in one bottle
= 485 × 200
= 97000 mg or 97 g
Mole is given by,
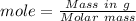
Moles in 97 g of ibuprofen =
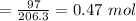
Moles of ibuprofen in a bottle of ibuprofen = 0.47 mol
b)
Number of molecules in one mole =
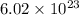
No. of molecules in 0.47 mol is,
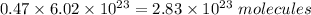
No. of molecules in a bottle of ibuprofen is
