Answer:
3CaSO₄ + 2AlCl₃ → Al₂(SO₄)₃ + 3CaCl₂
Step-by-step explanation:
The reaction expression is given as:
_CaSO₄ + _AlCl₃ → _Al₂(SO₄)₃ + _CaCl₂
To balance the reaction above, let us a mathematical approach;
assign coefficients a, b, c and d to the species;
aCaSO₄ + bAlCl₃ → cAl₂(SO₄)₃ + dCaCl₂
Conserving Ca : a = d
S: a = 3c
O: 4a = 12c
Al: b = 2c
Cl: 3b = 2d
So;
let a = 1, d = 1, c =
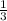 , b =
, b =
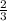
Multiply by 3 through;
a = 3, b = 2, c = 1 and d = 3
3CaSO₄ + 2AlCl₃ → Al₂(SO₄)₃ + 3CaCl₂