Answer: A)
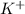 has 18 electrons.
has 18 electrons.
Explanation: Number of electrons in an atom equals to its atomic number. A cation is formed when electrons are lost from an atom and the anion is formed when electrons are gained by an atom.
Atomic number of K(potassium) is 19.
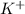 is formed when one electron is lost from K.
is formed when one electron is lost from K.
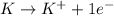
As one electron is lost from K to form
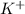 , the number of electrons will be 18.
, the number of electrons will be 18.
The atomic number of Cl(chlorine) is 17. In choice B, we have chlorine ion,
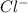 . Since it has -1 charge, the number of electrons for this ion will be 18 and not 17.
. Since it has -1 charge, the number of electrons for this ion will be 18 and not 17.
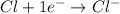
Atomic number of B(boron) is 5 means it has 5 electrons. In choice C, we have
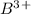 which is formed when 3 electrons are lost from B.
which is formed when 3 electrons are lost from B.
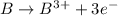
As 3 electrons are lost, there will only 2 electrons remaining.
Atomic number of Ra(Radium) is 88 means it has 88 electrons.
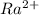 is formed when 2 electrons are lost from Ra.
is formed when 2 electrons are lost from Ra.
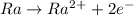
As two electrons are lost, there will be 86 electrons remaining.
So, the only correct choice is A.
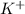 has 18 electrons.
has 18 electrons.