Answer: C) It increases with a decrease in the concentration of
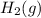 . D) It decreases with increase in the concentration of
. D) It decreases with increase in the concentration of
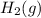 .
.
Explanation: According to Le Chatelier's principle, if an equilibrium reaction is disturbed, the reaction would try to undo the change imposed.
Thus if the concentration of the products in increased, the reaction would shift in a direction where the concentration of products is decreasing i.e in backward direction.
If the concentration of the products in decreased, the reaction would shift in a direction where the concentration of products is increasing i.e in forward direction.
When the concentration of either of the products, i.e
 or
or
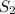 is increased, the reaction will shift in backward direction and the rate of forward direction decreases.
is increased, the reaction will shift in backward direction and the rate of forward direction decreases.