Answer:
1.016 M is the concentration of nitrate ions in a solution.
Step-by-step explanation:
Molarity of the solution is the moles of compound in 1 Liter solutions.
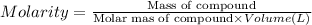
Mass of calcium nitarte = 25.0 g
Molar mass of calcium nitrate = 164 g/mol
Volume of the solution = 300 mL = 0.3 L ( 1 mL =0.001 L)
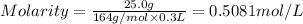
![[Ca(NO_3)_2]=0.5081 M](https://img.qammunity.org/2019/formulas/chemistry/high-school/wc3qb28dbwuy601ryy81in710c4fh37tnh.png)
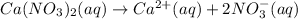
According to reaction 1 mole of calcium nitrate gives 1 mole of calcium ion and 2 moles of nitrate ions.
![[NO_3^(-)]=2* [Ca(NO_3)_2]=2* 0.5081 M=1.016 M](https://img.qammunity.org/2019/formulas/chemistry/high-school/nohu9w5uzf1ubwlr4kk4d3iwm8li3a58bo.png)
1.016 M is the concentration of nitrate ions in a solution.