Answer: The correct answer is 4-7.
Step-by-step explanation:
A non-metal is defined as the element which gains electron to attain stable electronic configuration and to attain a negative ion which is anion.

Non-metals are the elements which belong to Group 14 to Group 17.
The valence electronic configuration of the elements:
- Belonging to Group 14 =
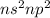
Number of valence electrons = 4
- Belonging to Group 16=
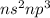
Number of valence electrons = 5
- Belonging to Group 16 =
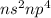
Number of valence electrons = 6
- Belonging to Group 17 =
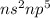
Number of valence electrons = 7
Hence, the correct answer is 4-7.