Answer: The correct answer is Option c.
Step-by-step explanation:
The equation for the given chemical reaction is:
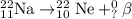
To know the missing term on the product side, total atomic number and mass number remains the same on both the sides.
Equating the terms of atomic number from both the sides, we get:
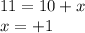
This decay is called as positron decay in which a proton gets converted to neutron and an electron neutrino and thus releases positron particles. This particle carries a charge of +1 units.
Hence, the correct answer is Option c.