Answer: 0.16 moles of
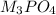 will be formed. Correct option is C.
will be formed. Correct option is C.
Step-by-step explanation: We are given 0.5 moles of Metal (I) halide and 0.2 moles of metal (II) phosphate. The reaction follows:
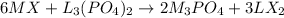
To calculate limiting reagent, we need to compare the actual ratio of moles to the stoichiometric ratio of moles of the reactants.
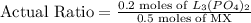
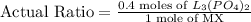
Now, the stoichiometric ratio:
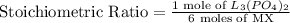
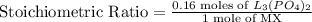
This means that at least 0.16 moles of
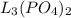 is required for every 1 mole of MX. As, the actual ratio is greater than the stoichiometric ratio, so
is required for every 1 mole of MX. As, the actual ratio is greater than the stoichiometric ratio, so
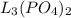 is present in greater amount. Therefore, MX is considered as the limiting reagent because it limits the formation of product.
is present in greater amount. Therefore, MX is considered as the limiting reagent because it limits the formation of product.
By Stoichiometry,
6 moles of MX produces 2 moles of
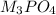
So, 0.5 moles of MX will produce =
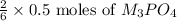
=
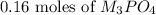
Hence, the correct option is C.