Hello!
What is the heat required to vaporize 650 grams of a liquid with a heat of vaporization of 723 joules/gram?
We have the following data:
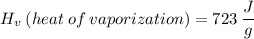
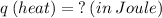
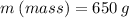
We apply the data to the formula, see:
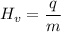
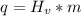
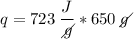
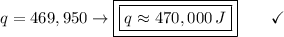
Answer:
E. 470,000 J
_______________________
I Hope this helps, greetings ... Dexteright02! =)