Answer: Option (D) is the correct answer.
Step-by-step explanation:
Polarity is defined as the presence of charges (positive or negative) present on the atoms of a compound or molecule.
For example, NaCl is a polar molecule because it has ionic bond whereas
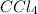 is a non-polar molecule.
is a non-polar molecule.
Also, it is known that like dissolves like that is, a polar molecule is soluble in a polar solvent whereas a non-polar molecule is soluble in a non-polar solvent.
When we stir, increase the surface area, and increase the temperature for a solution then rate of reaction also changes but molecular polarity does not affect the rate of a reaction.