Answer:
Hydrogen gas has the same number of molecules as 3.0 liter of nitrogen gas.
Step-by-step explanation:
Given volume of nitrogen gas at STP = 3 L
Moles of nitrogen gas = n
At STP, 1 mole of gas occupies 22.4 L of volume.
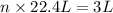
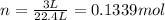
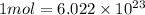 atoms/ molecules
atoms/ molecules
Number of nitrogen gas molecules:
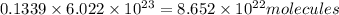
So, from the options, the gas with volume equal to the volume of nitrogen gas and will have moles equal to number of moles of nitrogen with which it will also have same number of molecules of gas.
Volume of fluorine gas = 6.0 l
Volume of hydrogen gas = 3.0 L
Volume of nitrogen gas = 4.5 L
Volume of chlorine gas = 4.5 L
Volume of hydrogen gas = Given volume o nitrogen gas = 3.0 L
Hydrogen gas has the same number of molecules as 3.0 liter of nitrogen gas.