here since container is rigid so we can say here volume must be constant
so here we can say by ideal gas equation
PV = nRT
here we know that
P = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
so here if volume is given constant
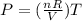
so we can say
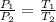
now we know that

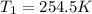
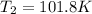
now from above equation we will have
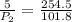
by solving above equation we will have
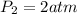
so final pressure will be 2 atm