Answer: The number of
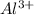 ions in given compound is
ions in given compound is

Step-by-step explanation:
We are given:
A chemical compound having chemical formula
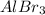
It is an ionic compound. This compound is formed by the complete transfer of electrons from 1 atom to another atom. The cation is formed by the loss of electrons by metals and anions are formed by gain of electrons by non metals.
It is formed by the combination of 1 aluminium ion and 3 bromine ions.
According to mole concept:
1 mole of an ionic compound contains
 number of ions.
number of ions.
So, 1 mole of
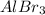 will contain
will contain
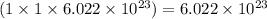 number of aluminium ions
number of aluminium ions
Hence, the number of
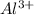 ions in given compound is
ions in given compound is
