Answer :
The test for
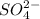 ion :
ion :
Procedure :
1) In a test tube, add 5-10 drops of aqueous solution of
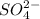 .
.
2) Add 1-2 ml of HCl solution and Barium chloride solution in the test tube.
The formation of white precipitate indicates the presence of
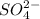 ion.
ion.
The chemical reaction is,
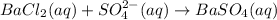
The White precipitate of
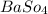 formed in this reaction.
formed in this reaction.