Here, the three different notation of the p-orbital in different sub-level have to generate
The value of azimuthal quantum number (l) for -p orbital is 1. We know that the magnetic quantum number
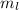 depends upon the value of l, which are -l to +l.
depends upon the value of l, which are -l to +l.
Thus for p-orbital the possible magnetic quantum numbers are- -1, 0, +1. So there will be three orbitals for p orbitals, which are designated as
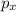 ,
,
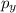 and
and
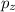 in space.
in space.
The three p-orbital can be distinguish by the quantum numbers as-
For 2p orbitals (principal quantum number is 2)
1) n = 2, l = 1, m = -1
2) n = 2, l = 1, m = 0
3) n = 2, l = 1, m = +1
Thus the notation of different p-orbitals in the sub level are determined.