Answer:
1. Hydrogen bonding
2. Dipole dipole interaction
3. London dispersion force
Step-by-step explanation:
Intermolecular forces refers to the force of attraction or force of repulsion between two molecules of same or other type.
In this case three types of Intermolecular forces acting:
1. Hydrogen bonding between O and H atom of different molecules.
2. Dipole dipole interaction between C and O atom because of great electronegative difference.
3. London dispersion force it is between two
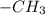 group of different molecules. It is also known as induced dipole force.
group of different molecules. It is also known as induced dipole force.
Hence, this is the required solution.