Answer : The mass of Butane needed is 29.946 g.
Solution : Given,
Molar mass of butane = 58.12 g/mole
Molar mass of carbon dioxide = 44.01 g/mole
Mass of carbon dioxide = 90.9 g
The Given net balanced chemical reaction is,
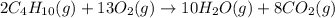
First we have to calculate the moles of carbon dioxide.
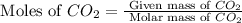
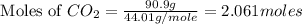
From the given chemical reaction, we conclude that
8 moles of
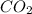 produced from 2 moles
produced from 2 moles
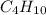
2.061 moles of
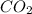 produced from
produced from
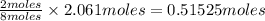 of
of
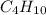
The moles of
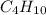 = 0.51525 moles
= 0.51525 moles
Now we have to calculate the mass of
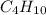 needed.
needed.
Mass of Butane = Moles of Butane × Molar mass of Butane
Mass of Butane = 0.51525 moles × 58.12 g/mole = 29.946 g
Therefore, the mass of Butane needed is 29.946 g.