As we know by the first law of thermodynamics
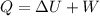
here we know that
Q = heat given to the system
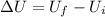
W = work done by the system
now here we can say

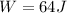
now we can say that heat will be given as
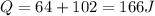
now here we can say that Jin does the error in his first step while calculation of change in internal energy as he had to subtract it while he added the two energy
So best describe Jin's Error is
B )For step 1, he should have subtracted 78 J from 180 J to find the change in internal energy.