Answer:
y = - 3x + 6
Explanation:
The equation of a line in slope- intercept form is
y = mx + c ( m is the slope and c the y- intercept )
Given
x - 3y = 3 ( subtract x from both sides )
- 3y = - x + 3 ( divide all terms by - 3 )
y =
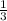 x - 1 ← in slope- intercept form
x - 1 ← in slope- intercept form
with slope m =
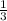
Given a line with slope m then the slope of a line perpendicular to it is
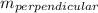 = -
= -
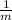 = -
= -
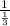 = - 3, then
= - 3, then
y = - 3x + c ← is the partial equation
To find c substitute (5, - 9) into the partial equation
- 9 = - 15 + c ⇒ c = - 9 + 15 = 6
y = - 3x + 6 ← equation of perpendicular line