Answer: The balanced chemical equation is
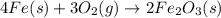
Step-by-step explanation:
Rust is reddish-brown coating on iron metal which is formed when iron comes in contact with air and moisture to form ferric oxide. The chemical formula for trust is
 and chemical name is ferric oxide.
and chemical name is ferric oxide.
The balanced chemical equation for the formation of rust follows:
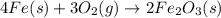
By Stoichiometry of the reaction:
4 moles of iron metal reacts with 3 moles of oxygen gas to produce 2 moles of ferric oxide.
Hence, the coefficients are 4, 3 and 2.