Answer : The partial pressure of
 is 98.825 kPa.
is 98.825 kPa.
Solution : Given,
Total pressure of hydrogen water vapor = 1 atm = 101.325 kPa (1 atm = 101.325 kPa)
Partial pressure of water vapor = 2.5 kPa
Dalton's law of partial pressure : It is defined as the total pressure exerted is equal to the sum of the partial pressure of individual gases.
Formula used :
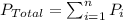
 = Total pressure
= Total pressure
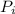 = Partial pressure of individual gases
= Partial pressure of individual gases

Now put all the given values in this expression, we get
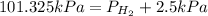
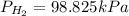
Therefore, the partial pressure of
 is 98.825 kPa.
is 98.825 kPa.