So,
% yield equals actual mass of product divided by theoretical mass of product times 100%.
1. First, write the balanced reaction.
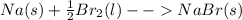
2. Since we are given the masses of both reactants, we need to do a Limiting Reactant calculation. Calculate the theoretical masses of product produced by each reactant, assuming excess of the other reactant.
a.
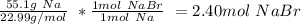
b.
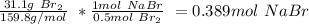
c. Bromine is the limiting reactant, and the theoretical mass of NaBr is:
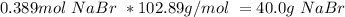
3. Now, we can calculate % yield.
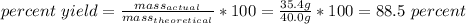
So, the percent yield is 88.5%.
Hope this helps!