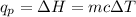Answer:
Pressure
Step-by-step explanation:
A coffee-cup calorimeter is essentially a constant-pressure calorimeter which is used to measure the enthalpy change (ΔH) in a solution. It comprises of a system of 2 nested styrofoam cups containing water which absorbs the heat from the reaction. A thermometer is inserted through the lid of the cup to record the changes in temperature.
Thus, the heat flow measured at constant pressure, q(p) is the enthalpy change (ΔH) of the reaction
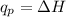
The amount of heat absorbed or evolved during a reaction is related to the mass of the substance(m), specific heat capacity (c) and the change in temperature, ΔT