Answer : The correct option is (B).
Explanation :
Body-centered cubic structure (bcc) :
Crystal structure : The manner in which the atoms or ions are spatially arranged.
In bcc arrangement, the atoms are present at eight corners of the cube and at the center of the cube. And the coordination number is 8.
Each unit cell has
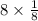 atoms at the corner and 1 full atom at the center of the cube.
atoms at the corner and 1 full atom at the center of the cube.
Therefore, the each unit cell has
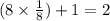 atoms.
atoms.
Image of bcc structure is shown below.