Answer:- 1.62 moles
Solution:- At constant temperature and pressure, volume is directly proportional to the moles of the gas.
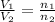
from given data,
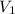 = 5.17 L,
= 5.17 L,
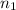 = 1.05 moles
= 1.05 moles
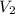 = 8.00 L,
= 8.00 L,
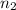 = ?
= ?
Let's plug in the values in the formula:
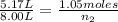
On cross multiply:
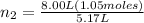
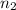 = 1.62 moles
= 1.62 moles
So, now the toy contains 1.62 moles of the air.