The law of conservation of mass states that mass can neither be created or destroyed, hence we expect product mass to equal reactant mass.
mass of propane + mass of oxygen = mass of carbon-dioxide + water
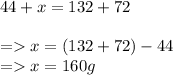
Using stochiometry, we first write a balanced the equation
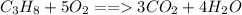
The ratio of propane to oxygen is 1:5, 1 mol propane reacts with 5 mol oxygen
First find the moles of propane using the mass of propane 44g and molecular mass of propane 44.1g/mol
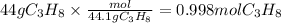
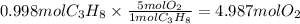
The grams of oxygen would be, using
 molecular mass 32 g/mol
molecular mass 32 g/mol
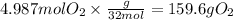
The answer rounded to 3 significant figures gives us
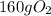 .
.