Answer: Option (d) is the correct answer.
Step-by-step explanation:
The given formula
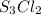 has three sulfur atoms and two chlorine atoms.
has three sulfur atoms and two chlorine atoms.
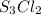 is a covalent compound as it is formed by sharing of electrons.
is a covalent compound as it is formed by sharing of electrons.
So, according to the nomenclature rule "tri" will be added before the name of cation because there are three atoms of cation and "di" will be added before the name of anion.
Therefore, we can conclude that the correct name of compound
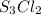 is Trisulfur dichlroide.
is Trisulfur dichlroide.