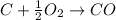Answer: incomplete supply of oxygen
Step-by-step explanation:
Combustion is a chemical reaction in which a fuel is reacted with excess supply of oxygen to result into complete oxidation of fuel to give carbon dioxide and water.
Example of complete combustion :
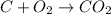
Incomplete combustion happens when the supply of oxygen is limited and thus fuel can not be completely oxidized , thus forming carbon monoxide.
Example of incomplete combustion :