Answer:
The pH of the solution is 2.26 .
Step-by-step explanation:
Mass of HCl = 200 mg = 0.200 g
1 mg = 0.001 g
Moles of HCl =

Volume of the solution ,V= 1 L
Molarity of HCl solution =
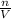
![[HCl]=(0.005479 mol)/(1 L)=0.005479 mol/L](https://img.qammunity.org/2019/formulas/chemistry/middle-school/y2z3mddzqvt35ifvgkaoufi5cwgkehiel9.png)
1 mole of HCk gives 1 mole of hydrogen ion and 1 mole of chloride ions.
![[HCl]=[H^+]=[Cl^-]=0.005479 mol/L](https://img.qammunity.org/2019/formulas/chemistry/middle-school/kegj6m3w2pf93peup28i44ynukvkdatvnq.png)
The pH of the solution is negative logarithm of hydrogen ion concentration:
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
![pH=-\log[0.005479 mol/L]=2.26](https://img.qammunity.org/2019/formulas/chemistry/middle-school/rcystzmn70um7zttn0ob89ce82h8n5tnym.png)
The pH of the solution is 2.26 .