The statement is incorrect because an atomic orbital is a mathematical function that is used to calculate the probability of finding an electron in a certain region of space. The location and energy of an electron is determined by a set of quantum numbers, n which represents the energy level of the electron, l which represents the angular momentum,
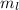 which represents magnetic quantum number and
which represents magnetic quantum number and
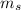 describes the spin of the electron. Therefore the electron can be found on s,p.d,f orbital based on this quantum numbers. So the atomic orbital gives a probable region where the electron can be found as opposed to it being a definite location of the electron.
describes the spin of the electron. Therefore the electron can be found on s,p.d,f orbital based on this quantum numbers. So the atomic orbital gives a probable region where the electron can be found as opposed to it being a definite location of the electron.